Viral outbreaks are not new; we have faced SARS, MERS, Nipah, Zika and several other viral outbreaks in the recent past. However, none has made the world stand still as COVID-19 has. The World Health Organization (WHO) has declared this outbreak a pandemic due to its high transmission rate; as of 2 April 2020, more than 950,000 cases have been reported, of which more than 48,000 patients have died. The number of cases and deaths continues to rise as it has spread to over 200 countries and territories, making it a global concern. A drug or vaccine that will help end this pandemic is needed.
COVID-19 was identified by pneumonia causalities at Wuhan, the epicenter of the outbreak. Though early cases were linked to a wet market in the Hubei Province, it is now understood that the virus spread through human-to-human transmission via droplets or direct contact; the infection is estimated to have a mean incubation period of 6.4 days and a basic reproduction number of 2.24–3.58. The major symptoms recorded in COVID-19 infected causalities include fever, cough, shortness of breath, chest tightness, and kidney failure.
COVID-19 is a beta coronavirus, similar to the human coronaviruses SARS and MERS. With this novel outbreak, there are now seven different strains of human coronaviruses (HCoVs): the 229E and the NL63 strains of HCoVs (alpha coronaviruses), OC43, HKU1, SARS, MERS, and COVID-19 HCoVs (beta coronaviruses).
As the world braces to fight the global pandemic through social distancing and country-wide lockdown, here’s a snapshot of advances in healthcare technologies that will help transform human lives affected by COVID-19:
Potential COVID-19 Treatment Avenues
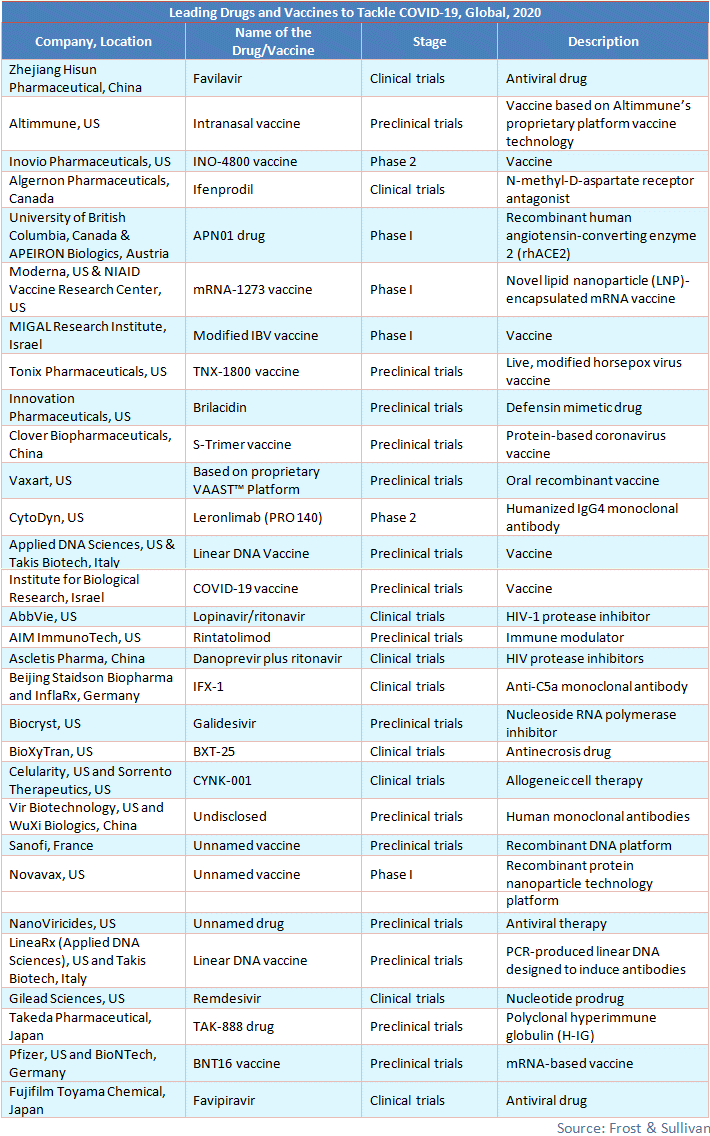
While governments and health authorities are deploying primary intervention to control the spread of COVID-19, pharmaceutical companies are focusing on identifying vaccines and drugs that will help combat the spread. They are repurposing already available drugs to combat COVID-19, such as Remdesivir, Chloroquine, Lopinavir/Ritonavir, and APN01 (ACE2 protein decoy).
According to WHO, some vaccines are currently being developed. In collaboration with Moderna, Inc., scientists from the National Institute of Allergy and Infectious Diseases (NIAID) have developed a vaccine called mRNA-1273, which is in Phase 1 trial and ready to be tested on study volunteers. Zydus Cadila is in the process of using the plasmid DNA vaccine and measles reverse-genetics technology to develop a vaccine. US-based Codagenix and the Serum Institute of India have partnered to develop a live-attenuated vaccine, which is in the pre-clinical testing phase and is likely to be launched by 2022. Inovio Pharmaceuticals, Inc., the company with a Phase-2 vaccine for COVID-19, plans to begin human clinical trials by April 2020 and deliver the INO-4800 vaccine by the end of the year.
More than 30 companies and healthcare institutes have stepped forward to develop a treatment for patients. The most prominent ones are mentioned below.
Viable treatments or vaccines must reach the market on time. Creating vaccines is an expensive and relatively time-consuming process as they have to go through several clinical trials before they can be approved by the US FDA. Governments that have placed orders for the COVID-19 vaccine may break their contracts if the virus gets phased out in another few months, just as with the SARS vaccine in the past. Frost & Sullivan is closely monitoring developments in this space.
Diagnostic Kits that could be Game-changers in COVID-19 Detection
Digital healthcare companies and start-ups are playing a major role in assisting and diagnosing COVID-19 by virtual care and providing telemedicine guidance. These measures can contain the spread of the virus by offering virtual care to patients quarantined at home.
Veredus Laboratories Pte Ltd, one of the leading solution providers of molecular diagnostics, has introduced a portable Lab-on-Chip kit for detecting COVID-19 in a single test. The kit can also be used to detect SARS and MERS-CoV. The detection kit uses the VereChip™ technology, which combines two molecular applications: polymerase chain reaction (PCR) and microarray. These two applications have high specificity and sensitivity to distinguish the COVID-19 strain from the SARS or MERS strains. Dr. Rosemary Tan, CEO of Veredus Laboratories, claims that the kit is capable of detecting the strain in a single test in two hours.
The US FDA recently granted regulatory approval to Thermo Fisher Scientific for the TaqPath COVID-19 Combo Kit, a test kit that could detect COVID-19 within four hours. As the kit has received emergency use authorization, it can be used by high-complexity CLIA labs.
On 13 March 2020, Roche received authorization from the US FDA to use Cobas SARS-CoV-2 test, an efficient and easy-to-administer test, which could deliver results in three-and-a-half hours.
Several diagnostic test developers have sought FDA authorization specifically for COVID-19: Co-Diagnostics for its Logix Smart™ Coronavirus Disease 2019 (COVID-19) test kit; Hologic for its Panther Fusion SARS-COV-2 assay; and Laboratory Corporation of America (LabCorp) for its COVID-19 RT-PCR test.
Sonovia Ltd., an Israeli start-up, says it has designed a mask that could block the virus and prevent secondary infection. The mask is made using ultrasonic irradiation to incorporate metal-oxide nanoparticles with antimicrobial property. The mask has been shipped to China to test its effectiveness against COVID-19.
This coronavirus outbreak might be the first public health threat that has seen the largely rhetorical 5G remote diagnosis being used. This would enable the lifting of the ban or restrictions on Zhongxing Telecommunication Equipment (ZTE) and Huawei 5G services in some countries.
COVID-19 is a growing global threat and various industry sectors are joining hands to combat this emergency. Early Frost & Sullivan analysis reveals that hydroxychloroquine and Remdesivir are likely to emerge as important treatment strategies for COVID-19. The diagnostic landscape will also see a sharp rise in “emergency use authorization” by global regulatory authorities such as the US FDA, as already witnessed in the cases of Mesa Biotech and Cepheid. This will enable early detection and timely management of COVID-19. As global healthcare experts unite to fight COVID-19 through technology innovations, Frost & Sullivan also commits itself to practicing social distancing, while closely tracking research developments and disruptions in this space.
Frost & Sullivan offers comprehensive services encompassing COVID-19:
Work Done by Frost & Sullivan on COVID-19:
To know more on Frost & Sullivan’s analysis on COVID-19 from across the globe, visit – https://www.frost.com/insights/covid19/.
Should you have any queries on the impact of COVID-19 across industries, or need more information or would like to schedule an interview/interaction with our spokespersons, please email Srihari Daivanayagam at Srihari.Daivanayagam@frost.com.